Our multi-modal investigational drug, efruxifermin (EFX), is designed to treat MASH holistically.
EFX has been engineered to mimic the biological activity of fibroblast growth factor 21 (FGF21), which regulates multiple metabolic pathways and cellular processes. By delivering sustained and balanced signaling through FGF21’s receptors in liver and adipose tissue, EFX has the potential to treat MASH by addressing all core drivers of disease progression.
EFX leverages the whole body to improve metabolic balance.
96-week analysis of our Phase 2b HARMONY study evaluating the safety and efficacy of EFX in patients with pre-cirrhotic MASH showed how balanced agonism of FGF21’s receptors has the potential to:
- Induce ≥1- and ≥2 stage improvements in fibrosis without worsening of MASH at 50mg and 28mg doses at rates significantly above placebo
- Provide sustained effects, with 92% and 83% of those with available week 96 biopsies whose fibrosis improved at week 24 in the 50mg and 28mg EFX groups, respectively, remaining responders compared to 40% for placebo
- Induce improvements across a range of metabolic biomarkers, including liver fat, enzymes, noninvasive fibrosis markers, glycemic control, lipoproteins, and body weight
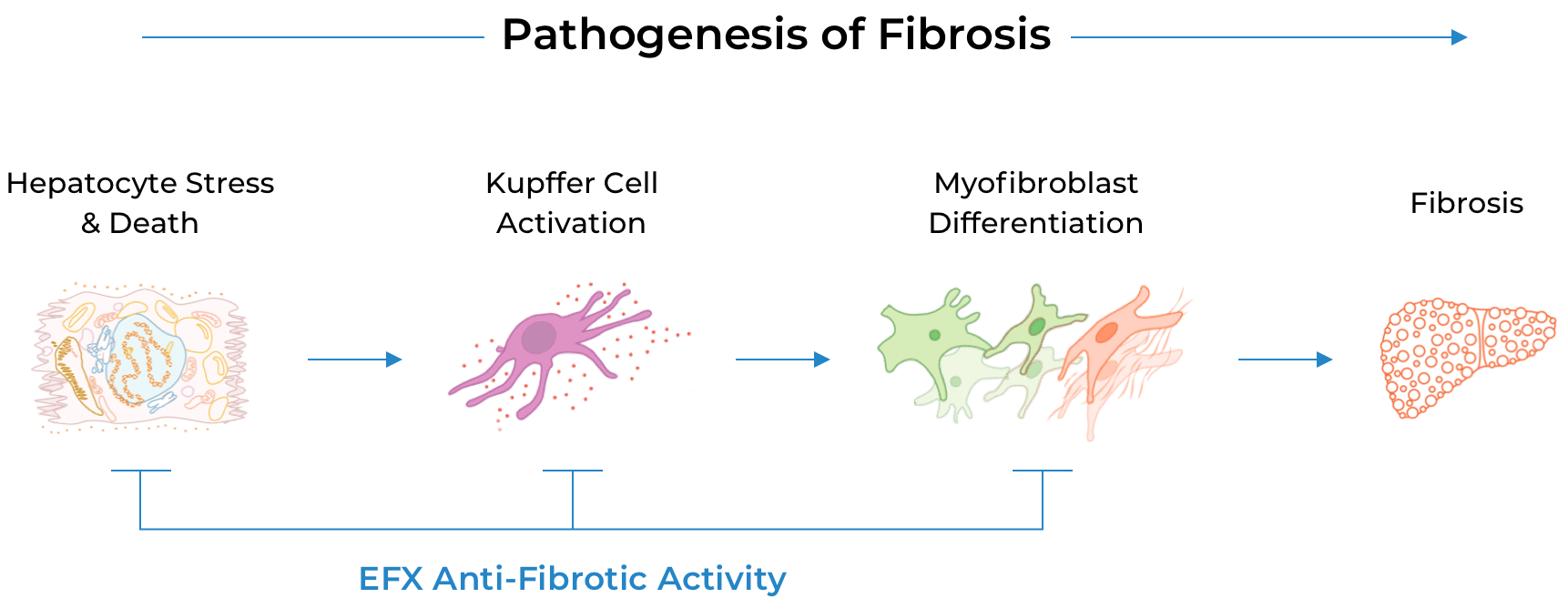
We believe effective treatment of MASH requires the reversal of fibrosis – particularly among patients with the most advanced fibrosis. We believe EFX has the potential to act at each step of the cascade that leads to fibrosis.
- 41% and 39% of patients in the 50mg and 28mg dose groups, respectively, demonstrated ≥1 stage improvement in fibrosis without worsening of MASH, double the placebo rate (20%)
- 76% and 47% of patients in the 50mg and 28mg dose groups, respectively, demonstrated MASH resolution without worsening of fibrosis, three to five times the placebo rate (15%)
- 41% and 29% of patients in the 50mg and 28mg dose groups, respectively, demonstrated fibrosis improvement AND resolution of MASH, six to eight times the placebo rate (5%)
EFX was also reported to be generally well tolerated. Gastrointestinal events, which were transient in nature, were the most frequent side effect.
These encouraging results pave the way for continued development of EFX, which we believe has the potential to be a foundational MASH monotherapy.

