We have designed a comprehensive clinical development program to deliver EFX, if approved, as quickly as possible to patients in need.
We believe our clinical trial data, which include some of the largest and most consistent effects reported to date in the MASH space, position EFX to be a potentially best-in-class treatment, if approved, for MASH. To date, data from a suite of completed and ongoing clinical studies have demonstrated that EFX has the potential to improve key indicators, including sustained improvements in liver fat and markers of liver injury, fibrosis, and key metabolic biomarkers.
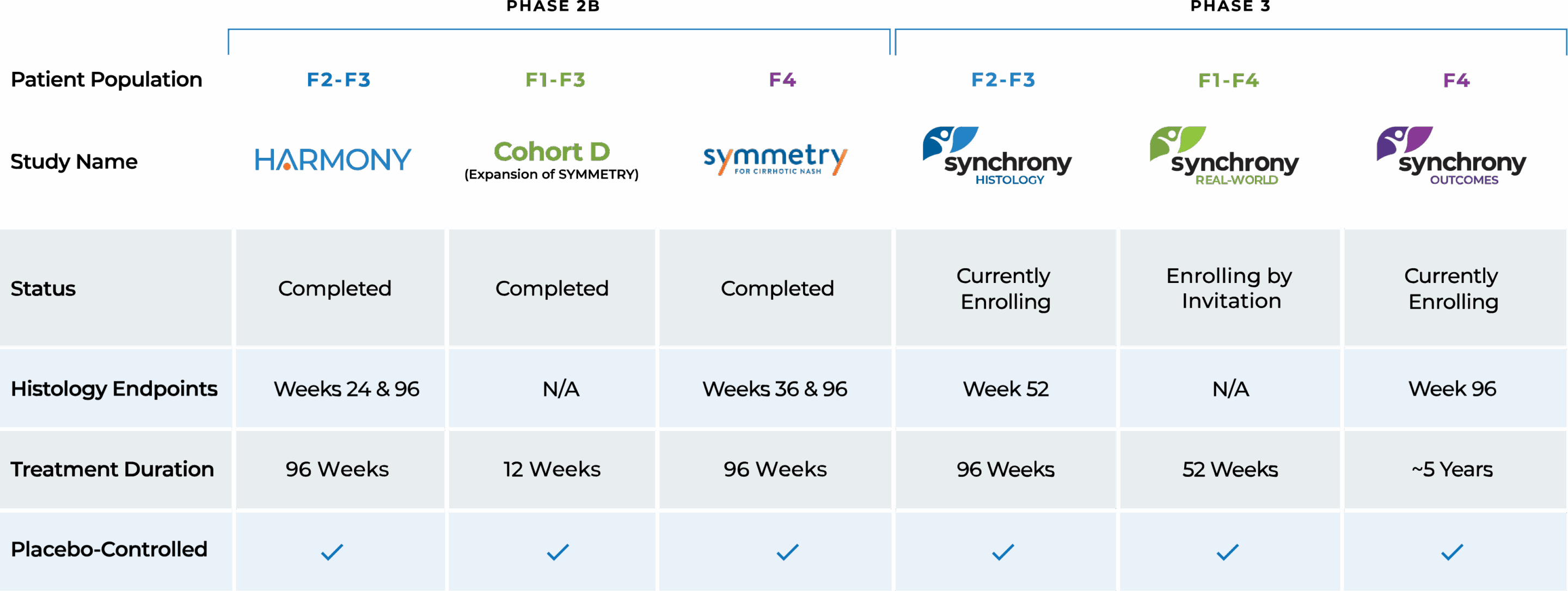
We currently are enrolling three clinical trials as part of our Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic (F2-F3) MASH and compensated cirrhosis (F4) due to MASH. The SYNCHRONY studies build on a robust Phase 2 program and have been designed in consultation with global regulatory authorities.
SYNCHRONY
STATUS: ongoing- SYNCHRONY Histology, which is evaluating the efficacy and safety of EFX in patients with biopsy-confirmed pre-cirrhotic (F2-F3) MASH;
- SYNCHRONY Real-World, which is assessing the safety and tolerability of EFX in patients with noninvasively diagnosed MASH or metabolic dysfunction-associated steatotic liver disease (MASLD) (F1-F4);
- SYNCHRONY Outcomes, which is evaluating EFX for the treatment of compensated cirrhosis (F4) due to MASH.
SYMMETRY
STATUS: completed