Our multi-modal investigational drug, efruxifermin (EFX), is designed to treat MASH holistically.
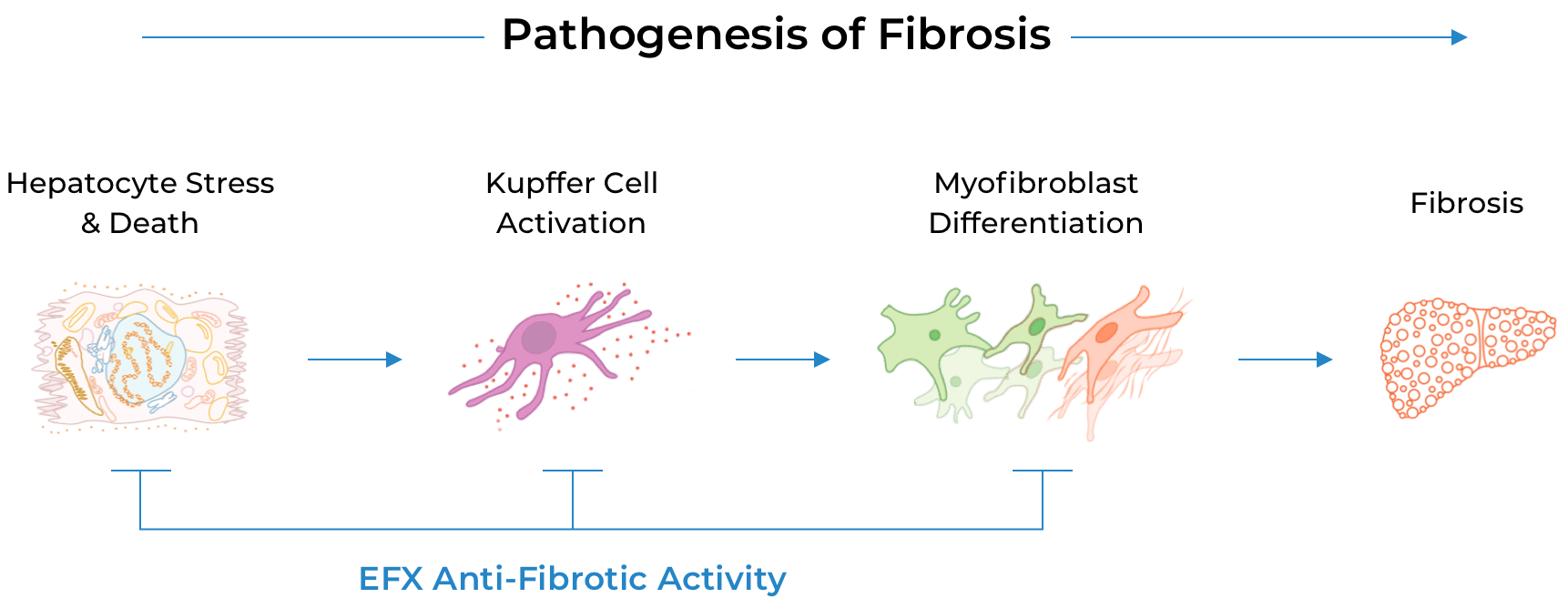
EFX has been engineered to mimic the biological activity of fibroblast growth factor 21 (FGF21), which regulates multiple metabolic pathways and cellular processes. By delivering sustained and balanced signaling through FGF21’s receptors in liver and adipose tissue, EFX has the potential to treat MASH by addressing all core drivers of disease progression.
EFX leverages the whole body to improve metabolic balance.
EFX currently is being evaluated in the Phase 3 SYNCHRONY program, which is composed of three clinical trials designed to support marketing applications for treatment of pre-cirrhotic MASH as well as compensated cirrhosis due to MASH.
The SYNCHRONY program builds on results from two 96-week Phase 2b clinical trials.
HARMONY Study
The first Phase 2b study evaluated the efficacy and safety of EFX in patients with pre-cirrhotic MASH, fibrosis stage 2 or 3 (F2-F3). The study met its primary endpoint of ≥1 stage improvement in fibrosis with no worsening of MASH after 24 weeks of treatment for both the 50 mg EFX (41%) and 28 mg EFX (39%) dose groups, compared to 20% for the placebo arm.
Secondary and exploratory endpoints showed EFX’s potential to sustain and deepen fibrosis improvement through 96 weeks of treatment, together with improvements in markers of liver injury and fibrosis, glycemic control, and lipoproteins. Notable findings at week 96 included:
- 75% (p<0.001) and 46% (p=0.07) of patients in the 50 mg and 28 mg EFX dose groups, respectively, experienced at least a 1-stage improvement in fibrosis without worsening of MASH, compared to 24% for placebo.
- 36% (p<0.01) and 31% (p<0.01) of patients treated with 50 mg and 28 mg EFX, respectively, experienced a 2-stage improvement in fibrosis without worsening of MASH—more than 10-fold the placebo rate of 3%.
- 68% and 40% of subsets of patients in the 50 mg and 28 mg EFX dose groups, respectively, who had F3 fibrosis at baseline experienced at least a 1-stage improvement in fibrosis without worsening of MASH, compared to 14% for placebo.
The fibrosis improvement observed after only 24 weeks with sustained and deepened response seen at 96 weeks, along with preclinical data, suggest that EFX has the potential to act directly as well as indirectly to reverse fibrosis—a feature that differentiates EFX from many other therapeutic mechanisms that act only indirectly.
SYMMETRY Study
The second and ongoing Phase 2b study evaluated EFX for the treatment of compensated cirrhosis (F4) due to MASH, providing additional evidence of EFX’s potential to reverse fibrosis, including among patients at greatest risk of progressing to liver failure. Although statistical significance was not achieved on the primary endpoint of fibrosis improvement at 36 weeks, an approximate doubling of effect size from weeks 36 to 96 underscored the benefit of longer EFX treatment for patients with compensated cirrhosis (F4):
- Among patients with baseline and week 96 biopsies, 39% of the 50mg EFX group (p<0.01) demonstrated ≥1 stage improvement in fibrosis with no worsening of MASH compared with 15% for placebo.
-
By ITT analysis, in which all participants with missing biopsies were imputed as non-responders, 29% of the 50mg EFX group (p<0.05) demonstrated ≥1 stage improvement in fibrosis with no worsening of MASH compared with 12% for placebo.
Additionally, among a subgroup of patients who were not taking GLP-1 at baseline (n=97), the rate of cirrhosis reversal for 50mg EFX was 45% (p<0.01), compared to 17% for placebo, indicating GLP-1-independent cirrhosis reversal.
The cirrhosis reversal observed by histological endpoints in the SYMMETRY study was corroborated by improvements in key noninvasive measures of liver fibrosis and injury.
EFX has been reported to be generally well-tolerated across five clinical trials of EFX. Most adverse events, or AEs, were mild or moderate. Diarrhea and nausea as well as injection site reactions and increased appetite were generally the most common AEs

